Our vision is to use routine healthcare data to improve outcomes for critically ill patients with co-existing cardiovascular disease.
We are a diverse multi-disciplinary collaboration including patients and their relatives, clinicians, epidemiologists, data scientists and triallists.
People with cardiovascular disease (such as previous heart attack, stroke, or peripheral vascular disease) have a heart which is vulnerable at rest. During critical illness, the oxygen demand of both the heart and the whole body increases significantly. However, for patients with CVD, they may be unable to increase the oxygen delivery to the heart, resulting in an oxygen supply-demand imbalance. This may lead to a heart attack. We have shown that approximately a quarter of critically ill patients with CVD have a heart attack whilst in ICU, which are associated with poorer longer term outcomes, and that very few of these are diagnosed by the clinical team.
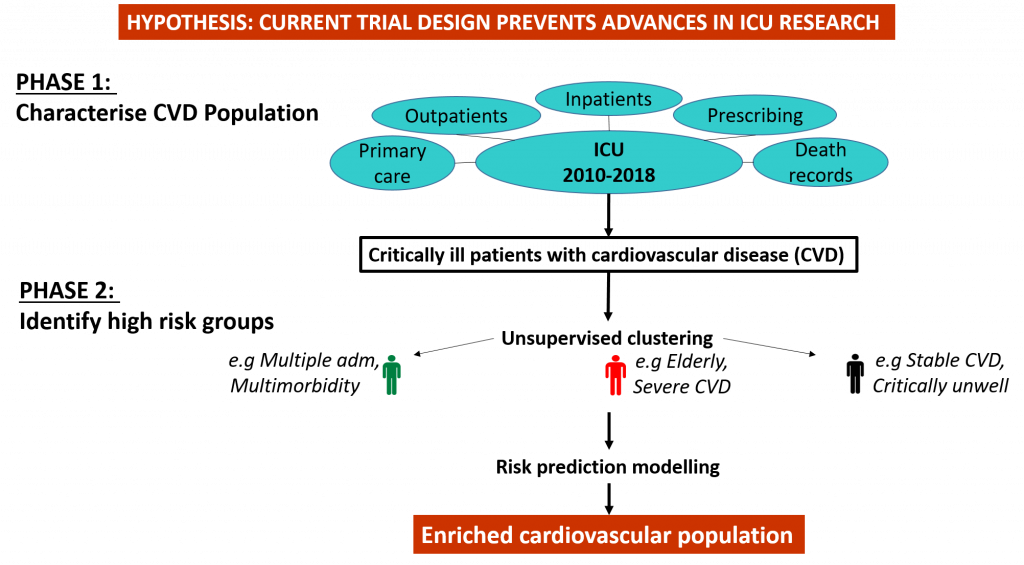
Our project of work proposes to use routine healthcare data to characterise this population of people at a national level for the first time, and identify patients who are both at risk of heart attacks, and who are having a heart attack. This will inform the novel design of a trial of higher vs lower blood concentration levels in this high risk population.
PHASE 1
We will describe critically ill patients with cardiovascular disease using data routinely collected in all Scottish and English hospitals for the first time. We will explore rates of cardiovascular events, such as heart attacks and stroke, and mortality, and look at where ICU fits into our patients’ health care trajectories.
PHASE 2
We will identify groups where cardiovascular risk is the greatest, and therefore where cardiovascular interventions are most likely to be beneficial.

PHASE 3
We will analyse de-identified vital signs data from critically ill patients’ bedside monitors, in order to assess damage to the heart muscle during patients’ ICU stay, and assess the association with longer term outcomes
PHASE 4
We will bring all the previous work together in the design of an adaptive trial. We will include patients at high risk of cardiovascular events, who may benefit most from the intervention. We will use the routine vital signs data as an intermediate outcome to feed back into the selection criteria for the trial to ensure that we are recruiting the right patients. Our outcomes will be informed by the work in phase one, in combination with patients and their relatives.
